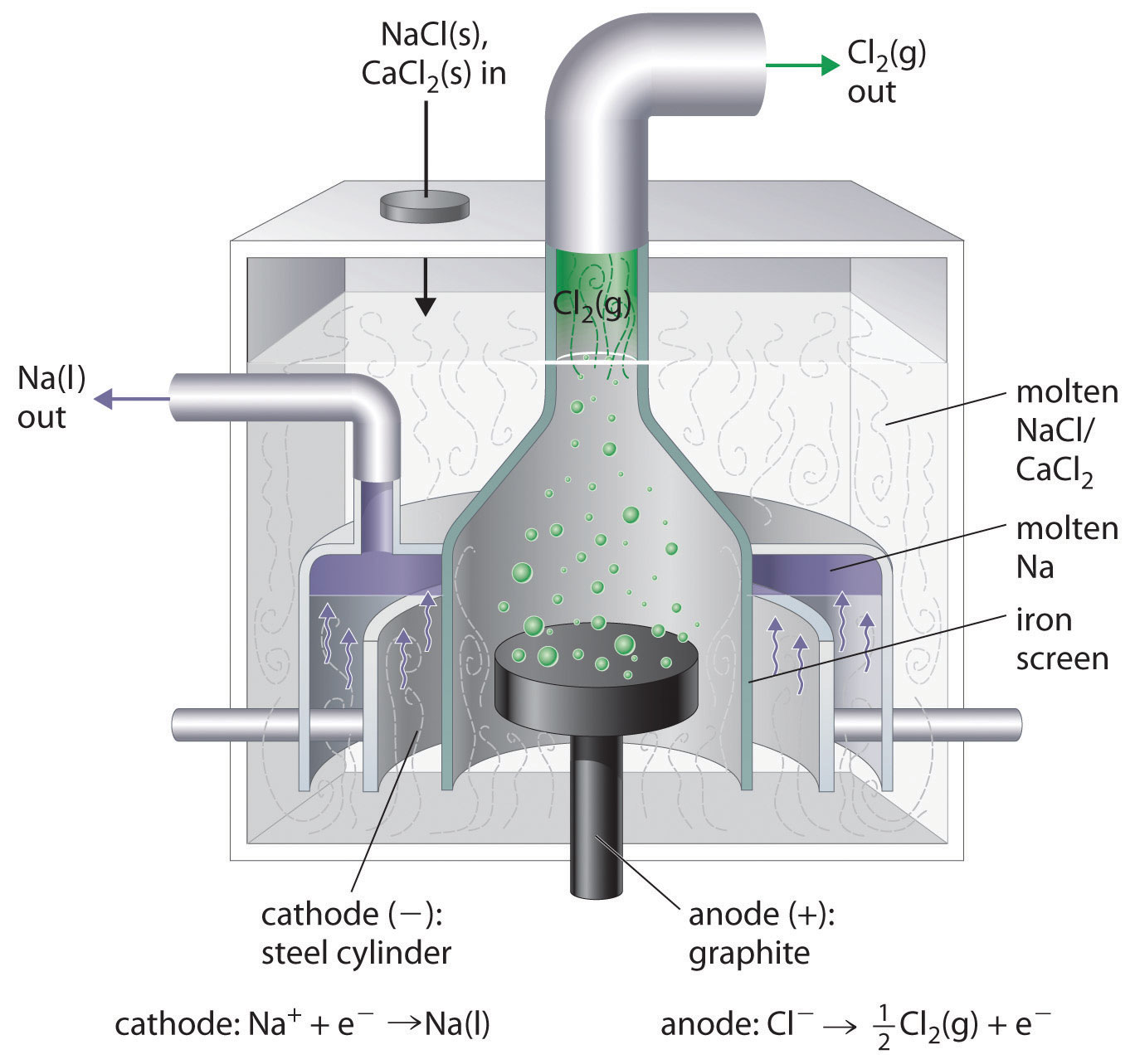
What Metals Can Be Used For Electrolysis . graphite is a good conductor, and is efficient in electrolysis. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they can be prepared only by the electrolysis. Tiny grains of black stuff are. the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. But it has a tendency to crumbling. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution.
from webmis.highland.cc.il.us
Tiny grains of black stuff are. reactive metals are extracted from their ores using electrolysis. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. graphite is a good conductor, and is efficient in electrolysis. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. Ionic compounds conduct electricity when molten or in solution. the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they can be prepared only by the electrolysis. But it has a tendency to crumbling.
Electrolysis
What Metals Can Be Used For Electrolysis Tiny grains of black stuff are. the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. Tiny grains of black stuff are. Ionic compounds conduct electricity when molten or in solution. graphite is a good conductor, and is efficient in electrolysis. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. But it has a tendency to crumbling. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. reactive metals are extracted from their ores using electrolysis. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they can be prepared only by the electrolysis.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte. Electrolysis of copper(II) sulfate What Metals Can Be Used For Electrolysis graphite is a good conductor, and is efficient in electrolysis. the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Tiny grains of black stuff are. reactive metals are extracted from their ores using electrolysis. Ionic compounds. What Metals Can Be Used For Electrolysis.
From webmis.highland.cc.il.us
Electrolysis What Metals Can Be Used For Electrolysis Tiny grains of black stuff are. But it has a tendency to crumbling. graphite is a good conductor, and is efficient in electrolysis. Ionic compounds conduct electricity when molten or in solution. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they can be prepared only by the electrolysis.. What Metals Can Be Used For Electrolysis.
From mungfali.com
Electrolysis Process Diagram What Metals Can Be Used For Electrolysis reactive metals are extracted from their ores using electrolysis. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. graphite is a good conductor, and is efficient in electrolysis. Ionic compounds conduct electricity when molten or in solution. electrolysis is used extensively in metallurgical processes,. What Metals Can Be Used For Electrolysis.
From keystagewiki.com
Electrolysis Key Stage Wiki What Metals Can Be Used For Electrolysis graphite is a good conductor, and is efficient in electrolysis. Ionic compounds conduct electricity when molten or in solution. Tiny grains of black stuff are. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. active metals, such as aluminum and those of groups 1 and. What Metals Can Be Used For Electrolysis.
From library.fiveable.me
Electrolysis Intro to Chemistry Study Guide 2024 Fiveable What Metals Can Be Used For Electrolysis But it has a tendency to crumbling. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. graphite is a good conductor, and is efficient in electrolysis. Tiny grains of black stuff are. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. the. What Metals Can Be Used For Electrolysis.
From www.youtube.com
Using Electrolysis To Extract Metals (GCSE Chemistry) YouTube What Metals Can Be Used For Electrolysis electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. But it has a tendency to crumbling. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they can be prepared only by the electrolysis. graphite is a good conductor, and is efficient in electrolysis.. What Metals Can Be Used For Electrolysis.
From www.youtube.com
Extraction of metals using Electrolysis N5 Chemistry Lesson 4 YouTube What Metals Can Be Used For Electrolysis the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. reactive metals are extracted from their ores using electrolysis. Tiny grains of black stuff are. But it has a tendency to crumbling.. What Metals Can Be Used For Electrolysis.
From www.thesciencehive.co.uk
Extraction and uses of metals* — the science sauce What Metals Can Be Used For Electrolysis But it has a tendency to crumbling. graphite is a good conductor, and is efficient in electrolysis. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they. What Metals Can Be Used For Electrolysis.
From chem2u.blogspot.com
chem2U Electrolysis of Copper(II) Sulphate What Metals Can Be Used For Electrolysis the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. Tiny grains of black stuff are. Ionic compounds conduct electricity when molten or in solution. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. But it has a tendency to crumbling. active metals, such as aluminum and. What Metals Can Be Used For Electrolysis.
From www.slideserve.com
PPT Extracting Metals by Electrolysis PowerPoint Presentation, free download ID6819415 What Metals Can Be Used For Electrolysis graphite is a good conductor, and is efficient in electrolysis. Ionic compounds conduct electricity when molten or in solution. But it has a tendency to crumbling. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. the metals commonly used in electroplating include cadmium, chromium, copper,. What Metals Can Be Used For Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry What Metals Can Be Used For Electrolysis But it has a tendency to crumbling. the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. Tiny grains of black stuff are. electrolysis is used extensively in metallurgical processes, such. What Metals Can Be Used For Electrolysis.
From philschatz.com
Electrolysis · Chemistry What Metals Can Be Used For Electrolysis electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Ionic compounds conduct electricity when molten or in solution. graphite is a good conductor, and is efficient in electrolysis. reactive metals are extracted from their ores using electrolysis. Tiny grains of black stuff are. active metals, such as aluminum and those of. What Metals Can Be Used For Electrolysis.
From wiredataheathowiu8.z22.web.core.windows.net
What Happens At The Cathode In Electrolysis What Metals Can Be Used For Electrolysis the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. reactive metals are extracted from their ores using electrolysis. Tiny grains of black stuff are. Ionic compounds conduct electricity when molten or in solution. But it has a tendency to crumbling. graphite is a good conductor,. What Metals Can Be Used For Electrolysis.
From www.aplustopper.com
What are the Uses of Electrolysis A Plus Topper What Metals Can Be Used For Electrolysis Tiny grains of black stuff are. graphite is a good conductor, and is efficient in electrolysis. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. reactive metals are extracted from their ores using electrolysis. active. What Metals Can Be Used For Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace What Metals Can Be Used For Electrolysis graphite is a good conductor, and is efficient in electrolysis. electrolysis is used extensively in metallurgical processes, such as in extraction (electrowinning) or purification. Tiny grains of black stuff are. But it has a tendency to crumbling. Ionic compounds conduct electricity when molten or in solution. reactive metals are extracted from their ores using electrolysis. the. What Metals Can Be Used For Electrolysis.
From www.goodscience.com.au
Extraction of Metals Good Science What Metals Can Be Used For Electrolysis graphite is a good conductor, and is efficient in electrolysis. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they can be prepared only by the electrolysis. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from.. What Metals Can Be Used For Electrolysis.
From www.youtube.com
Electrolysis of impure copper YouTube What Metals Can Be Used For Electrolysis the metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. the electrolysis can be done on a molten metal oxide (smelt electrolysis) which is used for example to produce aluminium from. reactive metals are extracted from their ores using electrolysis. active metals, such as aluminum and those of groups 1 and. What Metals Can Be Used For Electrolysis.
From circuitcoffeegirl89t1.z14.web.core.windows.net
Cathode Charge In Electrolysis What Metals Can Be Used For Electrolysis reactive metals are extracted from their ores using electrolysis. active metals, such as aluminum and those of groups 1 and 2, react so readily with water that they can be prepared only by the electrolysis. graphite is a good conductor, and is efficient in electrolysis. But it has a tendency to crumbling. electrolysis is used extensively. What Metals Can Be Used For Electrolysis.